COMPANY Categories: Contract Manufacturing Services and OEM

A provider of special patient-care products within the medical device industry, we offer global solutions to improve the quality of life! Qmedics AG is a young Swiss-based medical device manufacturer founded in 2008. Our long year experience in the medical device industry has made us very flexible and highly capable of finding the optimum solution for every customer specific needs.
Qmedics AG is a young Swiss-based medical device manufacturer founded in 2008. Our long year experience in the medical device industry has made us very flexible and highly capable of finding the optimum solution for every customer specific needs.Ever since our foundation, we have been based in North-East of Switzerland, a region famous for technical innovations, with an international character and a sense of global trading. At the beginning of QMEDICS’ existence, QMEDICS offered contract R&D, manufacturing as well as OEM manufacturing for medical devices. As OEM manufacturer we developed, produced and supplied a workhorse of a Nitinol stent QM1 with the matching balloon catheter. This was the pride of the company and became the base of the new product portfolio of today.

In 2017 QMEDICS became an independent company with the opportunity to be legal manufacturer of our own new product portfolio and to do direct sales.
Since the know-how, engineers and production of this design are still at QMEDICS we could improve and expand our product portfolio within a minimum of time. Today the total facility has an area of over 2800m2 of production space, storage areas and offices, including two clean rooms with a total surface of 1200m2. The facility houses sophisticated machinery, EtO-sterilization as well as packaging and labelling equipment. Finally, dedicated, motivated and highly experienced employees.

Customer Satisfaction
In support of our commitment to develop medical technology and services that contribute to successful outcomes for every patient, we have established global processes and procedures that allow customers to provide feedback. Information regarding device performance or services rendered is routinely reviewed so that customer feedback is a primary input to our continuous improvement mechanisms and to ensure that QMEDICS supports the global regulatory medical device vigilance systems.

Our Vision
At QMEDICS we work hard every day to make sure that our guiding principle “striving for excellence every day and constantly seeking to improve the services we offer” is reflected in everything we do
QMEDICS as a provider of special patient-care products within the medical device industry, we offer global solutions to improve the quality of life
As a medical device company, we embrace the changing healthcare environment and seek to identify and explore opportunities as they emerge. With the best patient care always in mind, we insist on the utmost in quality of products, character of employees and relationships with customers and colleagues. We commit to strengthen our position in the market and being a financially viable company through innovation and continuous improvement.

Our Mission
Understanding your business is our business.
Our goal is to design, develop, manufacture, and sell state-of-the-art medical products that will improve the lives of patients around the world
Partnership instead of competition.
We manufacture state-of-the-art products, environmentally-friendly and can flexibly adjust our production capacities to your order situation.
Sharing Expertise is our mission and a promise to customers and colleagues to share medical knowledge and expertise for the benefit of patients health.
Our experience and Swiss quality is your guarantee.
We are a community and we serve a community. Nothing we do stands alone, everything depends on cooperation and collaboration.
MAKE AN ENQUIRY:Our Services
Contract & OEM Manufacturing

QMEDICS contract manufacturing services consistently deliver quality assured products that meet tight specifications. Our extensive expertise and knowledge of international requirements helps to assure your product will be compliant with the harmonised standards and regional requirements. Our goal is to deliver customized products and personalized services, while providing flexibility and guidance to help our customers to achieve their goals in a timely, efficient, and cost-effective manner.
QMEDICS OEM and contracted manufacturing is a trusted partner with in-house stent and balloon development, manufacturing and clinical/regulatory capabilities.
We supply custom tailored products, solutions and technologies.
An overview of our Products Range
Unique Stent Characterization
The Qmedics new stent strategy is based on an innovative product technology, where every patient will get a stent with properties compliant with the lesion and the moveability of the artery.
The new Qmedics Stent-Delivery-System consists of 3 Variants "4F, 5F, 6F" deliverable in two types "PULL & FLEX".
The self-expanding Stent-Delivery-System is indicated to improve luminal diameter in patients with de novo- or restenotic symptomatic atherosclerotic lesions in peripheral arterial diseases.
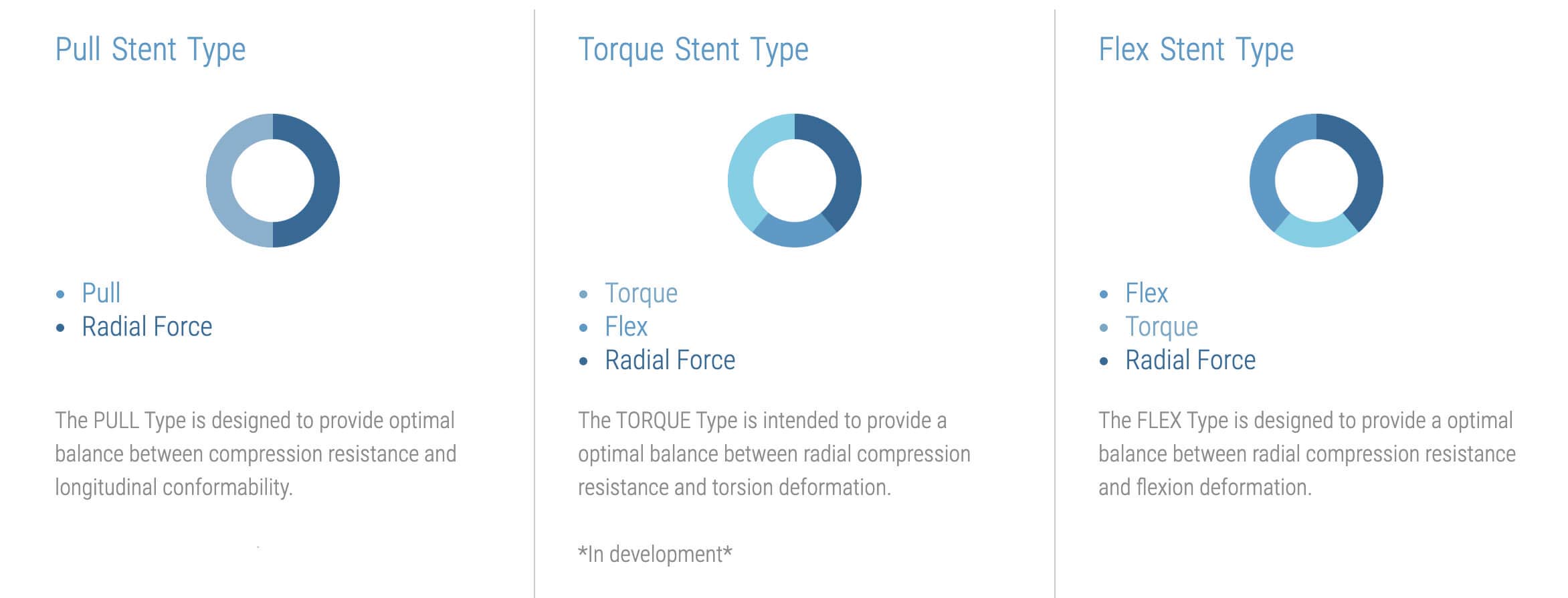

- Pull Stent Type
The PULL Type is designed to provide optimal balance between compression resistance and longitudinal conformability.
Stent Type Selection/ Indications:
For lesions assessed to benefit from treatment with additional radial force or less bend flexibility the Pull type may be selected.- Torque Stent Type
The TORQUE Type is intended to provide a optimal balance between radial compression resistance and torsion deformation. *In development*
The TORQUE Type is intended to provide a perfect balance between radial compression resistance and torsion deformation. The TORQUE Type is designed to avoid stent buckling and to provide an excellent patency within arteries subjected to torsion and radial compression.
Stent Type Selection/ Indications: *In development*
- Flex Stent Type
The FLEX Type is designed to provide a optimal balance between radial compression resistance and flexion deformation.
Stent Type Selection/ Indications
For lesions assessed to benefit from treatment with additional radial force or less bend flexibility the Pull type may be selected.

NOVA 4F
PTA Balloon Dilatation Catheter
The 418 BDC – PTA balloon The NOVA is a robust dual-lumen shaft balloon catheter with controlled compliance in diameters 2 to 6 mm and 40 to 220 mm length. The 2-lumen shaft combined with the low profile tip guarantees extreme flexibility and trackability. A broad size matrix will be delivered to treat a variety of vessel sizes and lesion types.
NAGA 5F
 PTA Balloon Dilatation Catheter
PTA Balloon Dilatation CatheterThe 518 BDC – PTA balloon The NAGA is a robust dual-lumen shaft balloon catheter with controlled compliance in diameters 4 to 7 mm and 40 to 220 mm length. Low profile opens access to small vessels and tortuous anatomy. The extra long usable of 175 cm length take you deeper into the anatomy and cross difficult lesions. A broad size matrix will be delivered to treat a variety of vessel sizes and lesion types.

MANATEE 6F
PTA Balloon Dilatation CatheterThe 635 BDC is a robust dual-lumen shaft balloon catheter with controlled compliance in diameters 4 to 10 mm and 40 to 220 mm length. Our 2-Lumen shaft has an inflation- and a guidewire lumen. Due to the balance between good trackability and pushability properties the catheter system as well owns short inflation- and deflation times.

ALBATROSS
Percutaneous Transluminal Sizing Balloon Catheter (PTA)The Percutaneous Transluminal Sizing Balloon Catheter is designed for patients suffering from cardiovascular defect (PFO, VSD, ASD). The Sizing Balloon Catheter is used for accurate measurements of the PFO, VSD and ASD. These measurements are essential to define the appropriate size of an occluder. The Sizing Balloon is intended for venous access. The catheter is introduced directly via the guide wire. A guide catheter is not necessary. The Sizing Balloon has an ultra thin wall thickness to gain super elastic behaviour. Pressure is defined by volume. *In development* Read More

DRACO 4F
NiTi Stent Delivery SystemThe Qmedics portfolio is based on a “back to the basic” concept, where each product has been carefully designed to be compliant with lesion charasteristics and movability of the artery.
The DRACO 4F self-expanding Peripheral Stent has an open cell peak-to-valley design and comes in different characteristics. Thin stent struts for lower restenosis rate. Two tantalum marker at each stent end for proper visibility. Stent diameter from 4 to 7 mm and a length from 40 to 120 mm. Marker band on outer shaft and a radiopaque tip for absolutely controlled stent deployment. Long lesion treatment with a single stent. Multifunctional braided catheter for optimal balance between pushability, trackability and accurate stent placement.
* Type Pull and Matrix-Extension under Development
* In submission
FALCOR 5F
 NiTi Stent Delivery System
NiTi Stent Delivery SystemThe Qmedics portfolio is based on a “back to the basic” concept, where each product has been carefully designed to be compliant with lesion charasteristics and movability of the artery.
The FALCOR 5F self-expanding Peripheral Stent has an open cell peak-to-valley design and comes in different characteristics. Thin stent struts for lower restenosis rate. Two tantalum markers at each stent end for proper visibility. Stent diameter from 5 to 8 mm and a length from 30 to 200 mm. Marker band on outer shaft and a radiopaque tip for absolutely controlled stent deployment. Long lesion treatment with a single stent. Multifunctional braided catheter for optimal balance between pushability, trackability and accurate stent placement. Read More
EXIST 6F
 NiTi Stent Delivery System
NiTi Stent Delivery SystemThe Qmedics portfolio is based on a “back to the basic” concept, where each product has been carefully designed to be compliant with lesion charasteristics and movability of the artery.
The EXIST 6F self-expanding peripheral stent has an open cell design. Two tantalum markers at each stent end for proper visibility. Stent diameter from 5 to 10 mm and a length from 30 to 200 mm. Marker band on outer shaft for accurate stent deployment. Unique, multi-step electro-polishing technology creates a smooth surface. Multifunctional braided catheter for optimal balance between pushability, trackability and flexibility and accurate stent placement. Read More
BUSTARD
 Valvuloplasty Balloon Catheter (PTV)
Valvuloplasty Balloon Catheter (PTV)The Bustard Percutaneous Transluminal Valvuloplasty (PTV) Catheter is engineered for Interventional Cardiology. The PTV balloon dilatation catheter is designed for maximum steering and tracking. The distal part of the balloon catheter is highly flexible combined with a soft distal tip for exceptional maneuverability. The Bustard dilatation catheter is a PTV Balloon Catheter with a 2-lumen shaft construction and a non-compliant balloon which can easily be placed over a guide wire. The Bustard is a particularly high pressure balloon catheter.
*In development* Read More
QMEDICS 418
 Balloon Dilatation Catheter
Balloon Dilatation CatheterThe Qmedics OTW 0.018” 4FR compatible PTA Balloon Catheter is a high performance balloon catheter for peripheral indication. The device features an ultra-low profile, semi-compliant balloon combined with a low profile tip and an improved shaft design, realize a better pushability for your ease of use especially in complex lesions, Tactile trackability for enhanced maneuvering and an excellent and fast deflation following the treatment.
QMEDICS 535
 Balloon Dilatation Catheter
Balloon Dilatation CatheterThe Qmedics OTW 0.035” 5FR compatible PTA Balloon Catheter is a high performance balloon catheter for peripheral indication. This 5Fr portfolio is the workhorse of balloons for the use for above the knee interventions . With superior cross and track, powerful dilatation, longer lengths, and smaller sheath size. Choose from the broad sizes offered to treat peripheral arterial disease (PAD) in a variety of vessel sizes and lesion types. Deliver high-pressure, targeted treatment to challenging lesions with the 535 Qmedics PTA balloon catheter, a balloon designed and engineered for peak performance at rated burst pressure. Choose the Qmedics 535 Balloon catheter for interventions that requires a product with balanced push and track, optimal balloon shape retention at rated burst pressure, and a best-in-class tip entry profile.
QMEDICS 635
 NiTi-Stent
NiTi-StentChoose the Qmedics 635 self-expending peripheral stent-system for its combined benefits of durability and deployement accuracy – all in a broad range of sizes.
Use the Qmedics 635 as an adjunct to percutaneous transluminal angioplasty “PTA” in the treatment of symptomatic peripheral vascular deseases. Its expanded indications represent a significant clinical milestone in achieving optimal treatment strategy for chronic pancreatitis patients suffering from bile duct strictures.

EasyX
Liquid EmbolicThe EasyXTM Liquid Embolics is a liquid polymer for embolization through small lumen delivery systems such as micro-catheters, occlusion balloon catheters and injection needles. After injection in liquid phase and upon contact with aqueous environment, the solvent rapidly dissipates in the surrounding tissue and the polymer precipitates in situ, providing a solid cast of the targeted vascular spaces.
NEW PRODUCT added to our Portfolio 31. July 2019
Our investors made it possible to expand our product portfolio with “Easyx“ a liquid Embolic that provides another treatment option for peripheral physicians - the start in Qmedics embolization portfolio, Qmedics embolization pipeline: occlusion balloon and an embolization plug
The EASYX™ Liquid Embolic is a new injectable, precipitating polymeric agent for the obliteration of vascular spaces through direct puncture or catheter access performed under X-ray guidance. The embolic liquid is iodinized Polyvinyl Alcohol (PVA) Polymer ether. Iodine groups are covalently grafted to the PVA polymer backbone, whereby a stable nondegradable polymer with the desired features is created. The resulting polymer is dissolved in Dimethyl Sulfoxide (DMSO).
EASYX™ is CE-marked since December 2016 for the use in peripheral vasculature. The safety and efficacy of EASYX™ embolization liquid for the percutaneous treatment of vascular lesions, i.e. embolization of varicocele, type II endoleaks, portal vein before surgery, active peripheral bleeding or angiomyolipoma (AML) was proven in a clinical trial EASYX-1.EASYX-1: A Multicenter Study on Safety and Efficacy of Easyx Liquid Embolization Agent Used in Five Separate Indications. Publication is planned very soon.


Second clinical trial for the extention of the EASYX indications:
Prospective, Multicenter, Multinational Study to Assess the Safety and Performance of the Easyx Liquid Embolic in Intracranial Interventions IDEALE Study.
This is a Prospective Study to Assess the Safety and Performance of the Easyx Liquid Embolic in Intracranial Interventions.- Email: anita.patteet@qmedics.chAddress: Winterthurerstrasse 708
8247
No Records Found
Sorry, no records were found. Please adjust your search criteria and try again.
Google Map Not Loaded
Sorry, unable to load Google Maps API.
MAKE AN ENQUIRY:


